Hyaluronic Acid: A Driver of Innovation in Health Products
루 론 산 (HA) is a naturally occurring glycosaminoglycan widely distributed throughout the extracellular matrix and various tissues다. As a fundamental biological component, it performs multiple physiological functions including regulating cellular behaviour, providing hydration and lubrication, and supporting tissue repair, thereby playing a crucial role in maintaining tissue homeostasis and biological function.
Benefiting from its unique viscoelasticity, excellent biocompatibility, and degradability, hyaluronic acid has become a vital raw material for numerous applications within the biomedical field. It is commonly utilised in the development of products such as ophthalmic surgical aids, post-operative anti-adhesion materials, skin wound repair formulations, drug delivery systems, and tissue engineering scaffolds.
As a supplier of hyaluronic acid raw materials, 녹색 봄 Technology strictly adheres to product quality standards, committed to providing customers with high-purity, multi-specification, safe and reliable hyaluronic acid powder. This supports downstream enterprises in innovation and application within the healthcare, skincare, and biomaterials sectors.
1 Hyaluronic Acid: Structure, Properties, and Its Application Value in Health Products
1.1 Structural Characteristics of Hyaluronic Acid and Its Advantages as a Functional Raw Material
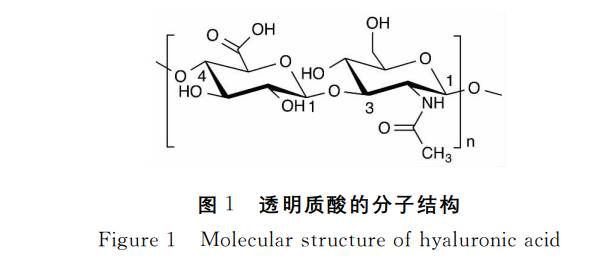
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan (GAG) widely distributed within the extracellular matrix and various human tissues. Its chemical structure comprises a high-molecular-weight linear polysaccharide formed by alternating units of D-glucuronic acid and N-acetyl-D-glucosamine linked via specific glycosidic bonds.This unique structure endows it with exceptional viscoelasticity, high moisture-retaining capacity, and excellent biodegradability, alongside outstanding biocompatibility and stability.
Unlike most glycosaminoglycans, hyaluronic acid is a non-sulphated polysaccharide capable of independent existence without relying on covalent protein cross-linking. This characteristic offers vast potential across multiple application domains. Presently, owing to its remarkable physicochemical and functional properties, hyaluronic acid has become a core raw material for developing innovative products across skincare, ophthalmology, joint health, and tissue engineering.
As a supplier of hyaluronic acid raw materials, 녹색 봄 Technology consistently provides clients with high-purity, multi-specification HA products meeting stringent quality standards, empowering enterprises to achieve technological breakthroughs and product upgrades in pharmaceuticals, skincare, and biomaterials.
1.2 Exceptional Hydrophilicity and Rheological Properties: The Material Foundation Empowering Hyaluronic Acid Product Innovation
Hyaluronic Acid (HA) is a white, amorphous solid with no distinctive odour. It possesses exceptional hydrophilic properties, rapidly dissolving in water to form solutions with unique rheological characteristics, yet remains insoluble in most organic solvents.Its molecular structure features regularly arranged hydrophilic groups interacting with hydrophobic regions to form a stable helical conformation. In aqueous solutions, HA chains extend and intertwine, creating a continuous three-dimensional network capable of effectively trapping substantial moisture. Research indicates HA can absorb up to 1000 times its own weight in water, earning it recognition as one of nature's most potent moisturising agents.
Even at low concentrations, hyaluronic acid solutions exhibit pronounced pseudoplasticity—a 1% solution assumes a gel-like state, demonstrating excellent flow and injectability under pressure alongside superior lubrication and tissue separation properties. This renders it suitable for applications demanding sophisticated rheological behaviour.
As a hyaluronic acid raw material supplier, Green Spring Technology is dedicated to providing high-purity hyaluronic acid ingredient solution across multiple molecular weight ranges. Their stable and reliable physicochemical properties enable broad application in skincare, medical devices, cosmeceuticals, and biomaterials, empowering clients to develop high-quality end products that combine innovation with practical value.
1.3 Hyaluronic Acid: Empowering Innovative Product Development Through Multi-Functional Properties
As a naturally occurring moisturising molecule with environmental responsiveness, hyaluronic acid (HA) dynamically adjusts its moisture-binding capacity according to ambient humidity, effectively maintaining hydration equilibrium within tissues and cells. Within the skin, it synergises with components like collagen to construct a highly hydrated extracellular matrix, significantly enhancing skin elasticity and structural support.
Beyond its exceptional moisturising properties, hyaluronic acid demonstrates free radical scavenging capabilities, assisting the body in maintaining oxidative stress equilibrium during metabolic processes. Within joint health, as a key component of synovial fluid, HA exhibits unique viscoelastic properties: providing lubrication during low-speed movements (such as walking) to reduce joint friction; acting as a buffer during high-speed or high-impact activities (such as running) to effectively disperse mechanical stress;and under weight-bearing conditions, it forms a gel-like structure to assist in pressure relief.
Hyaluronic acid also plays multiple roles in tissue repair. It participates in extracellular matrix reconstruction while aiding key processes including immune regulation, angiogenesis, and cell migration. Different molecular weights of hyaluronic acid perform distinct functions across repair stages: high-molecular-weight products provide structural support for cell migration, while low-molecular-weight fragments help stimulate fibroblast activity and tissue remodelling.
As a specialised supplier of hyaluronic acid raw materials, Green Spring Technology consistently provides clients with multi-molecular weight specifications, high-purity, and premium-quality hyaluronic acid ingredients. This fully supports innovation and product upgrades across diverse fields including skincare, joint health products, and tissue engineering materials, empowering enterprises to develop more competitive biomaterial solutions.
2 Applications of Hyaluronic Acid and Its Derivatives in Biomedicine
Hyaluronic acid and its derivatives demonstrate extensive application potential in biomedical materials due to their unique biocompatibility and rheological properties. Academic research categorises its functions into five primary applications:
(1) Viscous Support: Protecting sensitive tissues and creating working space during microsurgery, such as in ophthalmic procedures;
(2) Tissue Filling: Used for soft tissue support and volume restoration, such as in skin and vocal cord augmentation;
(3) Physical Separation: Isolates tissue surfaces post-surgery or trauma to facilitate effective interface separation;
(4) Lubrication Supplementation: Applied in joint-related fields to enhance lubrication and improve movement comfort;
(5) Surface Protection: Forms a protective layer on tissue surfaces to maintain a moist environment, supporting normal repair processes.
2.1 Innovative Applications and Technical Support of Hyaluronic Acid in Ophthalmology
As a natural component of the ocular vitreous humour, hyaluronic acid (HA) plays a vital role in ophthalmic surgery and eye health products.In precision procedures such as cataract surgery and intraocular lens implantation, hyaluronic acid is extensively employed as a surgical adjuvant. It effectively compensates for temporary vitreous fluid depletion, acting as a viscoelastic agent to protect the corneal endothelium, maintain anterior chamber space, buffer mechanical stress, and provide surgeons with a clear, stable operating field.
In the field of dry eye relief, hyaluronic acid leverages its exceptional water-retaining properties and rheological characteristics to enhance tear film stability and alleviate ocular dryness. It is extensively incorporated into artificial tears and eye care products as a functional ingredient.
Green Spring Technology, underpinned by rigorous quality control systems, supplies ophthalmic-grade high-purity, multi-molecular-weight hyaluronic acid raw materials. These products comply with relevant medical device standards, exhibit outstanding biocompatibility and batch consistency, and are dedicated to supporting corporate R&D innovation and commercial applications in premium ophthalmic medical devices and ocular health products.
2.2 Hyaluronic Acid Revolutionises Dermal Fillers Market, Driving Facial Rejuvenation
Hyaluronic acid (HA), a naturally occurring moisturising component within the human body, is a key substance for maintaining skin hydration and elasticity. With advancing age, HA gradually diminishes within the skin, closely linked to reduced moisturising capacity and altered skin condition. Possessing exceptional viscoelasticity, malleability, biocompatibility, and degradability, hyaluronic acid has become an indispensable biomaterial in skincare and medical aesthetics.
Within facial rejuvenation products, hyaluronic acid-based fillers are extensively utilised for tissue support and volume restoration, effectively diminishing wrinkles, reshaping contours, and reinstating facial fullness. According to the International Society of Aesthetic Plastic Surgery (ISAPS), these products rank among the most favoured choices in non-surgical aesthetic procedures.

Green Spring Technology is dedicated to supplying the beauty and skincare industry with high-purity, multi-molecular-weight hyaluronic acid raw materials, strictly adhering to quality and safety standards to empower enterprises in developing more effective and reliable innovations for facial rejuvenation and skincare.
2.3 Hyaluronic Acid Pioneers Post-operative Anti-adhesion and Wound Repair Material Innovation
Postoperative tissue adhesion represents a common surgical challenge, with its prevention and management emerging as a key focus for advanced medical device development. Leveraging its exceptional biocompatibility, tunable physicochemical properties, and biodegradable safety profile, hyaluronic acid (HA) demonstrates significant application potential in this field. It forms absorbable barrier films at postoperative tissue interfaces, effectively reducing adhesion risks while modulating local cellular behaviour and collagen organisation to establish a microenvironment conducive to normal repair.
Within trauma repair applications, hyaluronic acid is extensively employed as a matrix material for functional wound care products due to its superior hydration properties, film-forming capabilities, and controlled-release functionality. Industry innovation has yielded advanced designs such as ‘dual-layer hyaluronic acid repair materials’—where the upper layer provides mechanical barrier protection while the lower layer can carry active ingredients to promote wound healing and tissue regeneration.
Green Spring Technology is dedicated to providing clients with a comprehensive range of medical-grade hyaluronic acid raw materials, encompassing products of varying molecular weights and modification types. These cater to the development needs of high-end medical devices such as anti-adhesion membranes and wound repair dressings. Through stringent quality control, stable supply, and professional support, we empower enterprises to accelerate the implementation of innovative medical materials, delivering safer and more reliable solutions for patients.
2.4 Hyaluronic Acid Empowering Innovative Drug Delivery Systems
Owing to its exceptional biocompatibility, flexible modifiability, and innate targeted recognition capabilities, hyaluronic acid (HA) has emerged as a pivotal material in novel drug carrier development, demonstrating vast potential for enhancing therapeutic efficacy while reducing side effects. Through functional sites such as carboxyl and hydroxyl groups, hyaluronic acid can undergo diverse functional modifications to construct efficient, stable, and intelligent delivery systems, significantly improving intracellular drug delivery efficiency and tissue selectivity.
Green Spring Technology, leveraging its mature R&D and production platform, has launched a series of pharmaceutical-grade hyaluronic acid raw materials. These encompass diverse molecular weight ranges and functional group modifications, offering high purity and excellent batch-to-batch consistency. They comprehensively support the development and industrial application of innovative drug delivery systems such as liposomes, nanoparticles, and hydrogels.
3 전망
Since its first successful medical application in the mid-20th century, hyaluronic acid (HA) has undergone over six decades of global research and industrialisation. Its unique rheological properties and diverse physiological functions have established it as an indispensable core component in modern biomedical materials.
Presently, ongoing breakthroughs in modification techniques are driving increasingly active innovation in HA derivatives, continually introducing novel solutions across medical, skincare, and biomaterial fields. Despite significant advances in structural design, functional modification, and end-use applications, hyaluronic acid continues to demonstrate substantial scientific and industrial potential. Mechanistic research and material performance optimisation remain actively pursued globally.
As a trusted supplier of hyaluronic acid raw materials, Green Spring Technology remains dedicated to providing customers with high-purity, multi-specification hyaluronic acid and its derivative products, comprehensively serving diverse high-end applications including skincare, medical devices, drug delivery, and tissue engineering. Moving forward, we shall intensify R&D innovation and cross-sector collaboration, partnering with industry stakeholders to drive technological breakthroughs and product advancements in cutting-edge fields. This commitment aims to deliver safer, more effective biomaterial solutions for the global market.
Contact us today at helen@greenspringbio.com or WhatsApp: +86 13649243917 for specialised product specifications and quotations.
참조
[1] 마이어 K, 팔머 JW다. J Biol Chem, 1934년, 107: 629~634.
[2] 발라즈에아, 로랑 TC, Jeanloz RW,다. 바이오켐제이 (Biochem J, 1986년, 235: 903~903.
[3]Weigel PH, 하스콜 VC, 지난 밤에 M. J Biol Chem, 1997년, 272: 13997~14000.
[4] 코간 G, 용매 L, 스턴 R, Gemeiner P. 바이오 테크놀 렛 (Biotechnol Lett), 2007년, 29일: 17~25.
[5]Csoka AB, Frost기, 스턴 R다. 매트릭스 바이올 (Matrix Biol), 2001년, 20: 499~508.
[6] 데 볼레 K, 글로가우 R, 코노 T, 네이선 M, 테젤 A, 로카-마르티네즈 J-X, 팔리왈 S, Stroumpoulis D에 있다. 피부과 Surg, 2013년, 39대:1758~1766.
[7] 테르메르 C, 썰맨 JP, 사이 먼 JC다. Trends Immunol, 2003년, 24: 112~114.
-
Prev
Hyaluronic Acid: A Multifunctional Natural Ingredient to Boost Your Product Innovation
-
다음
Varying Molecular Weights of Hyaluronic Acid Drive Innovation in Cosmetics and Wellness Products


 영어
영어 프랑스
프랑스 스페인
스페인 러시아
러시아 한국
한국 일본
일본





